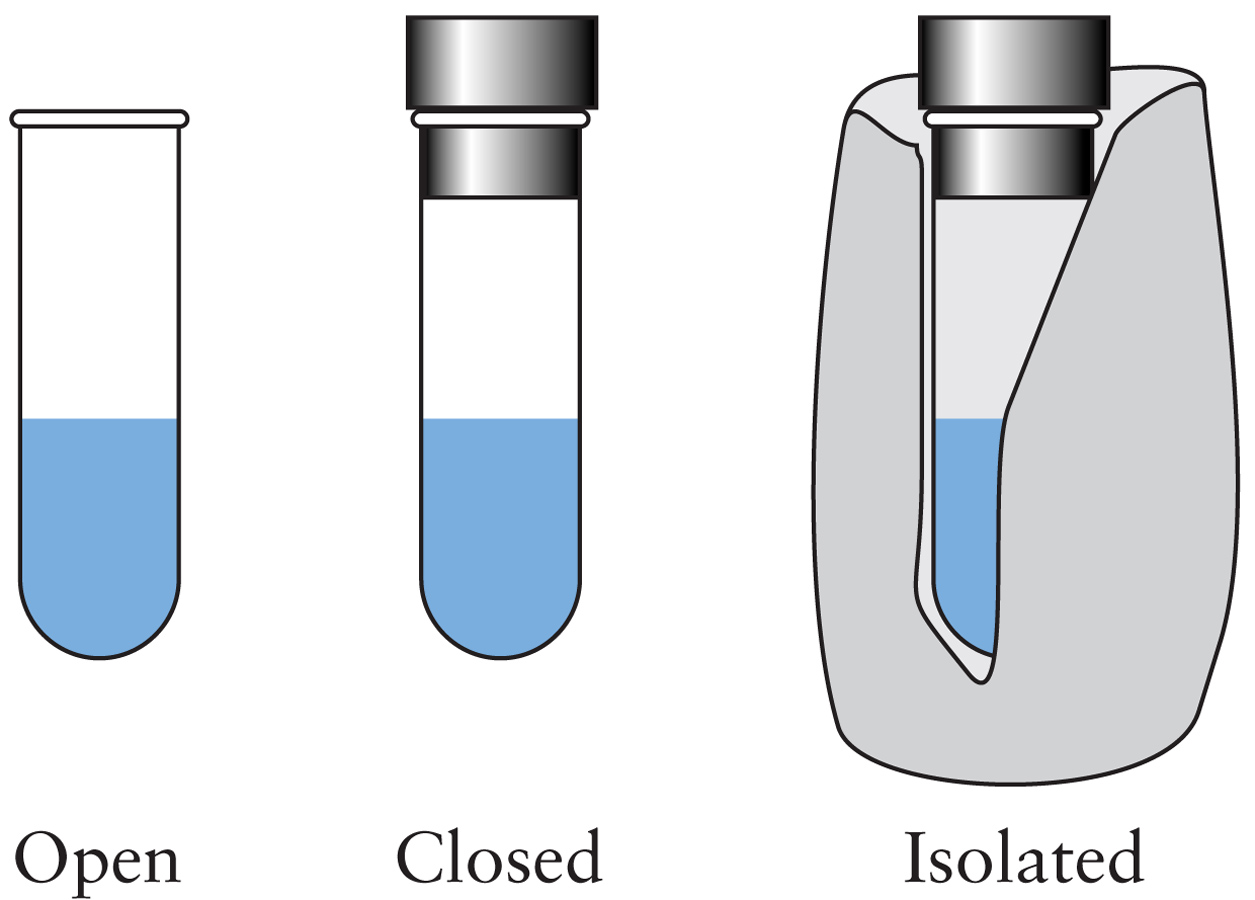
Laws of Thermodynamics
Underlying principles that govern all energy changes.
|
|
|
 |
First Law Statements:
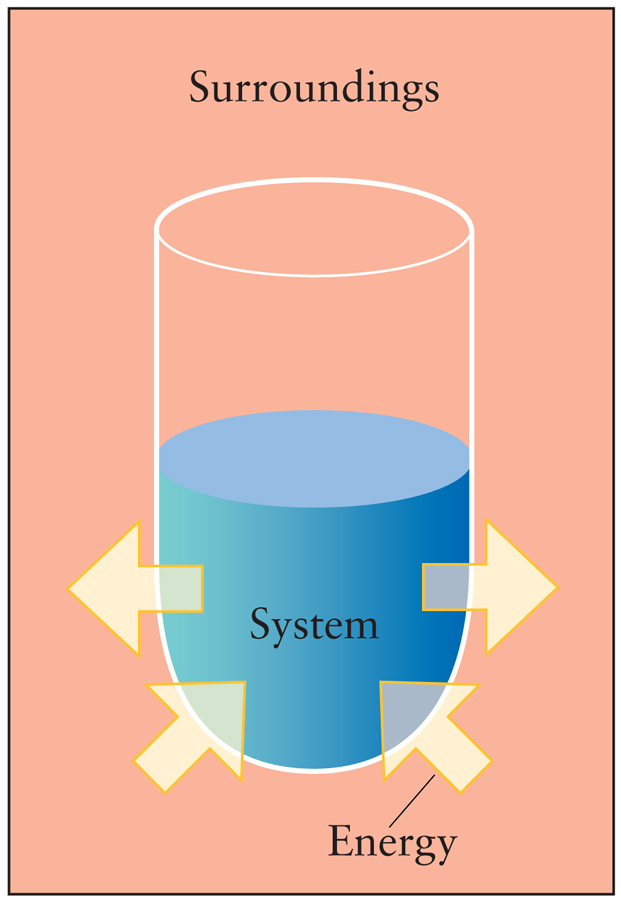
- Energy is conserved. - The energy of the universe is constant. - The change in the energy of the universe is zero. ΔEuniverse = 0 - The sum of energy changes in the system and surroundings is zero. ΔEuniverse = ΔEsystem + ΔEsurroundings = 0
- For an isolated system: ΔEsystem = 0 - For a closed system: ΔEsystem = q + w - For a closed system at constant volume with only PV work: ΔEsystem = qv
|
|
|
|