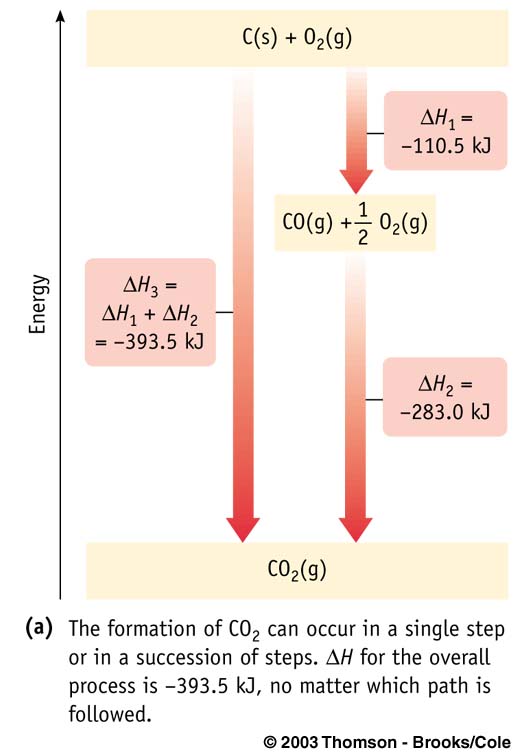
Hess's Law
The Reaction Enthalpy
, ΔHrxn, is the sum
of the enthalpies of any sequence of reactions (at the same temperature and pressure)
into which the overall reaction can be divided.
|
|
||
|
Using Hess's Law:
|
||
|
Reverse reaction
|
<–––> | Change sign of ΔH |
|
Multiply reaction by a number
|
<–––> | Multiply ΔH by same number. |
|
Add reactions
|
<–––> | Add ΔH's |
|
|
||
|
Why does this work ? |