Standard Reaction Enthalpy
The enthalpy change for a chemical reaction in which all reactants and products are in their standard states at a specified temperature.
ΔHorxn
Systematic way to compare energy changes of reactions.
Can be written in terms of the standard enthalpies of formation of products minus reactants (Hess's Law).
ΔHorxn
= Σ ni ΔHof
(products) – Σ nj ΔHof
(reactants)
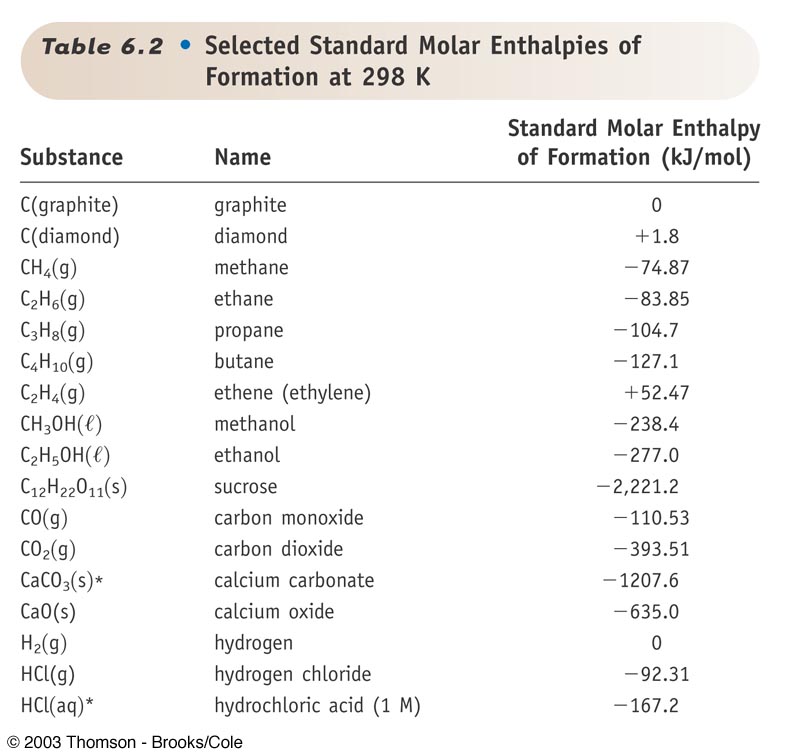
Calculate from tables of reference data.
|
|
 |
|
|
|
Example: CaCO3(s) + heat –––> CaO(s) + CO2(g)
|
|
ΔHorxn
= ΔHof
(CaO, s) + ΔHof
(CO2, g) – ΔHof
(CaCO3, s)
|
|
|