Bond Enthalpy
The energy required to break a chemical bond in the gas phase at constant pressure.
Endothermic always !
|
|
|
 |
 |
|
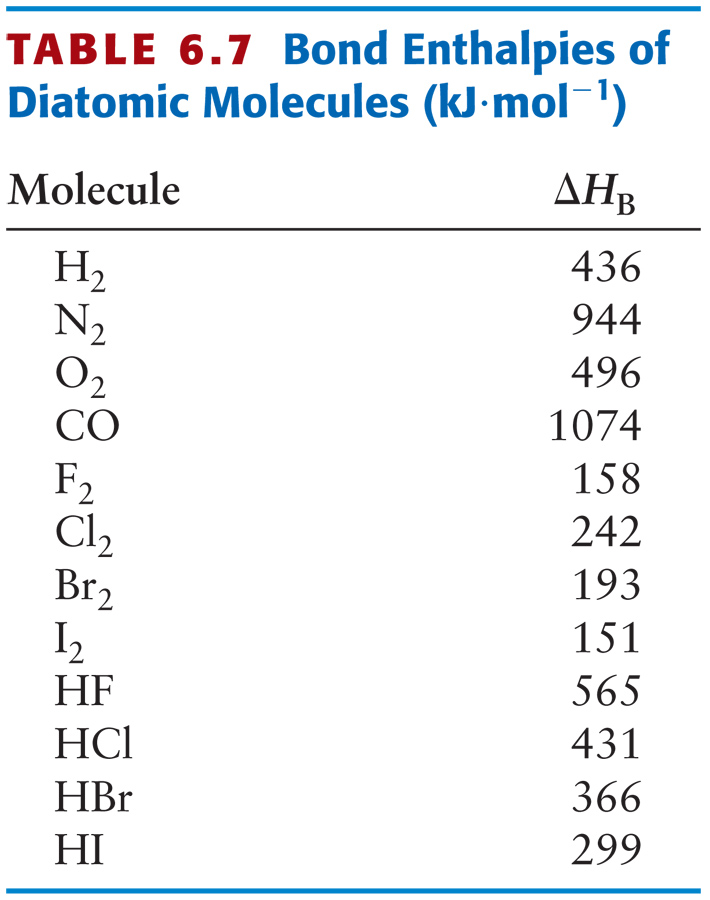
Actual values at 298 K
|
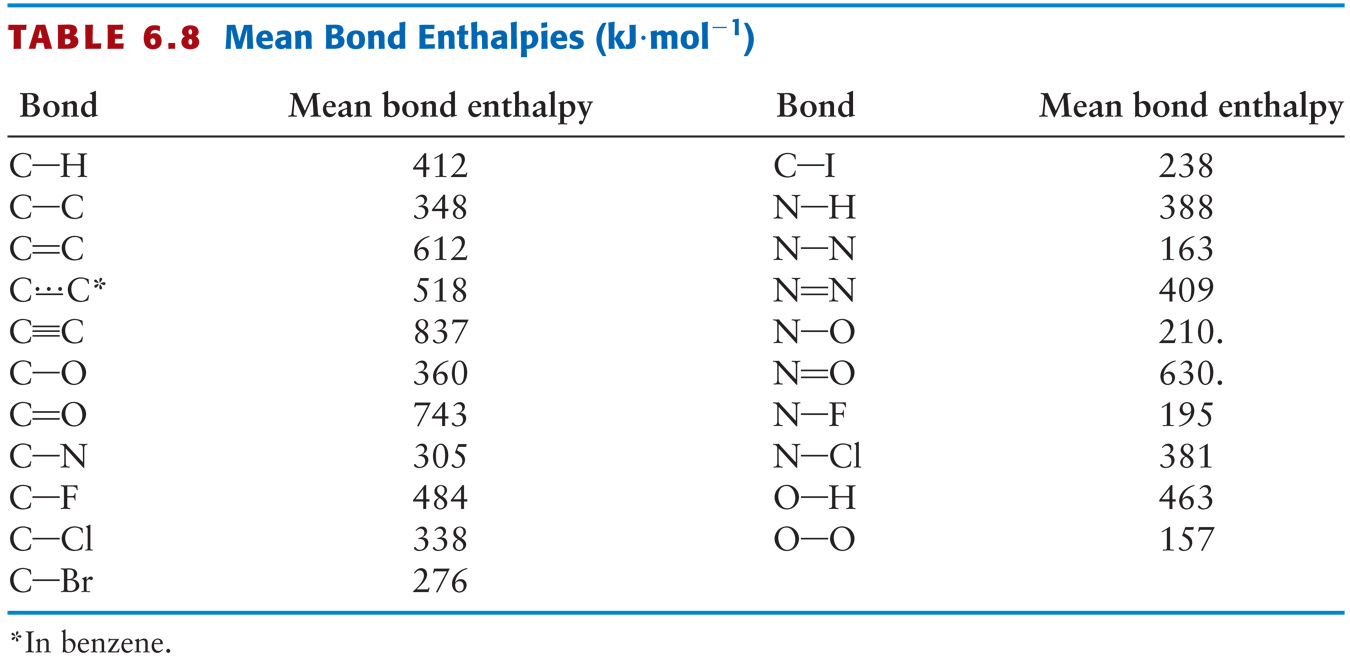
Average Values
|
|
|
|
ΔHorxn
Measure of relative bond strengths of reactants and products
|
|
||
|
Break
Make |
bonds of reactants
bonds of products |
Energy IN
Energy OUT |
|
|
||
Can estimate ΔHorxn
as sum of all reactant bonds broken minus all product bonds formed (another use of Hess's Law).
ΔHorxn
= Σ nBE
(reactants) – Σ nBE
(products)
|
|
||
|
If
|
Reactant bonds stronger than product bonds
|
1. Endothermic
2. Exothermic |
|
|
||
|
Example: CO2(g) + 4H2(g) –––> CH4(g) + 2H2O(g)
|
||
| ΔHorxn ~ 2 BE(C=O) + 4 BE(H–H) – 4 BE(C–H) – 4 BE(O–H) | ||
|
|
||