Calorimetry
Measurement of heat absorbed or evolved during a chemical or physical change.
Usually determined by measuring the temperature change
of a substance with a known heat capacity (often water).
– qchange = qwater + qcalorimeter
= (mCs(water) + Ccalorimeter)ΔT
|
|
||
|
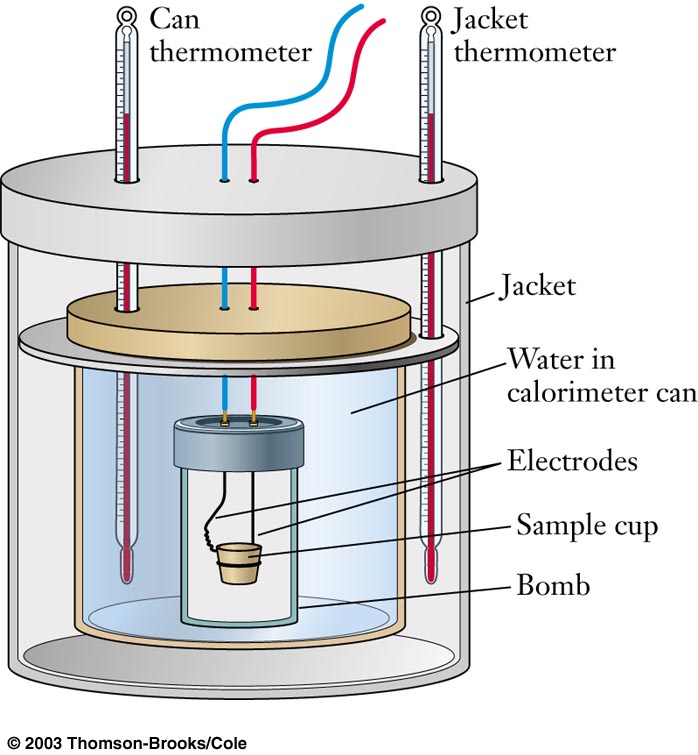
Bomb
|
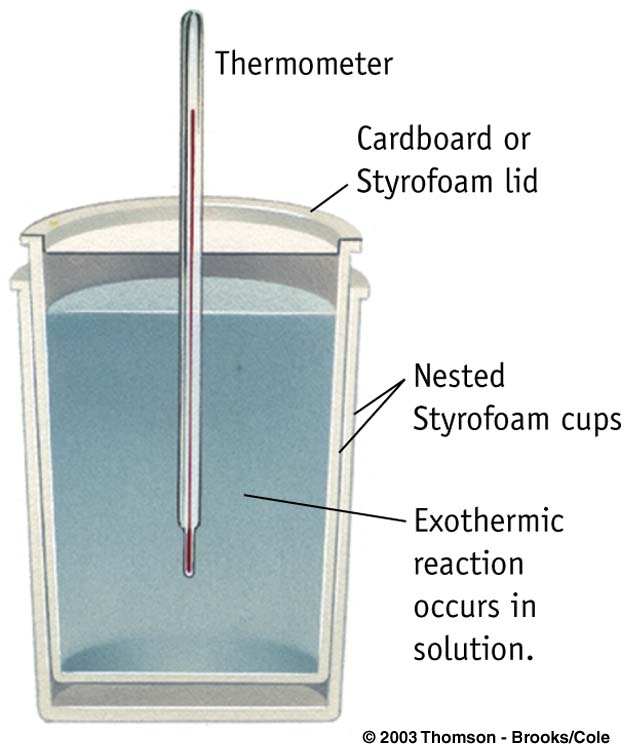
Coffee cup
|
|
|
Constant Volume
|
Constant Pressure
|
|
|
w = –Pext ΔV = 0
|
w = –Pext ΔV
|
|
|
q = ΔU
|
q = ΔH
|
|
|
|
ΔH = ΔU + PΔV
"add back" E used to expand |
|
|
Combustion reactions
Ccalorimeter large |
Specific Heats
Solution Phase reactions Ccalorimeter small |
|
 |
 |
|
|
|
||