Internal Energy
Sum of all the microscopic forms of E.
Sum of all Kinetic and Potential Energies of the molecules of a system.
U
(or E)
Hyper Physics link
Does not include translation or rotation of body as a whole.
Cannot measure total. Can only measure ΔU
|
|
|
 |
First Law Statements:
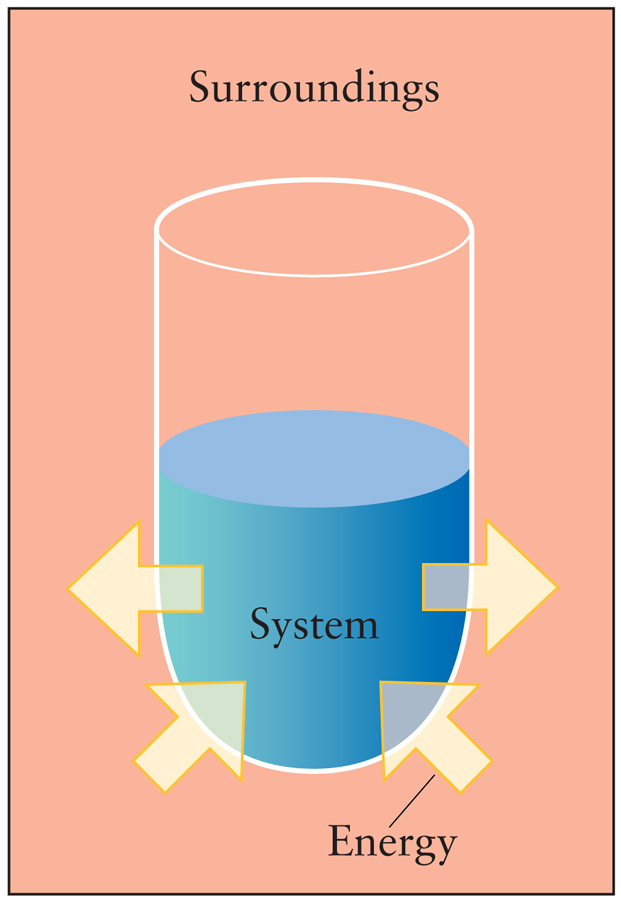
- A thermodynamic system can store or hold energy. This Internal Energy is conserved. - The internal energy of the universe is constant. - The change in the internal energy of the universe is zero. ΔUuniverse = 0 - The sum of energy changes in the system and surroundings is zero. ΔUuniverse = ΔUsystem + ΔUsurroundings = 0
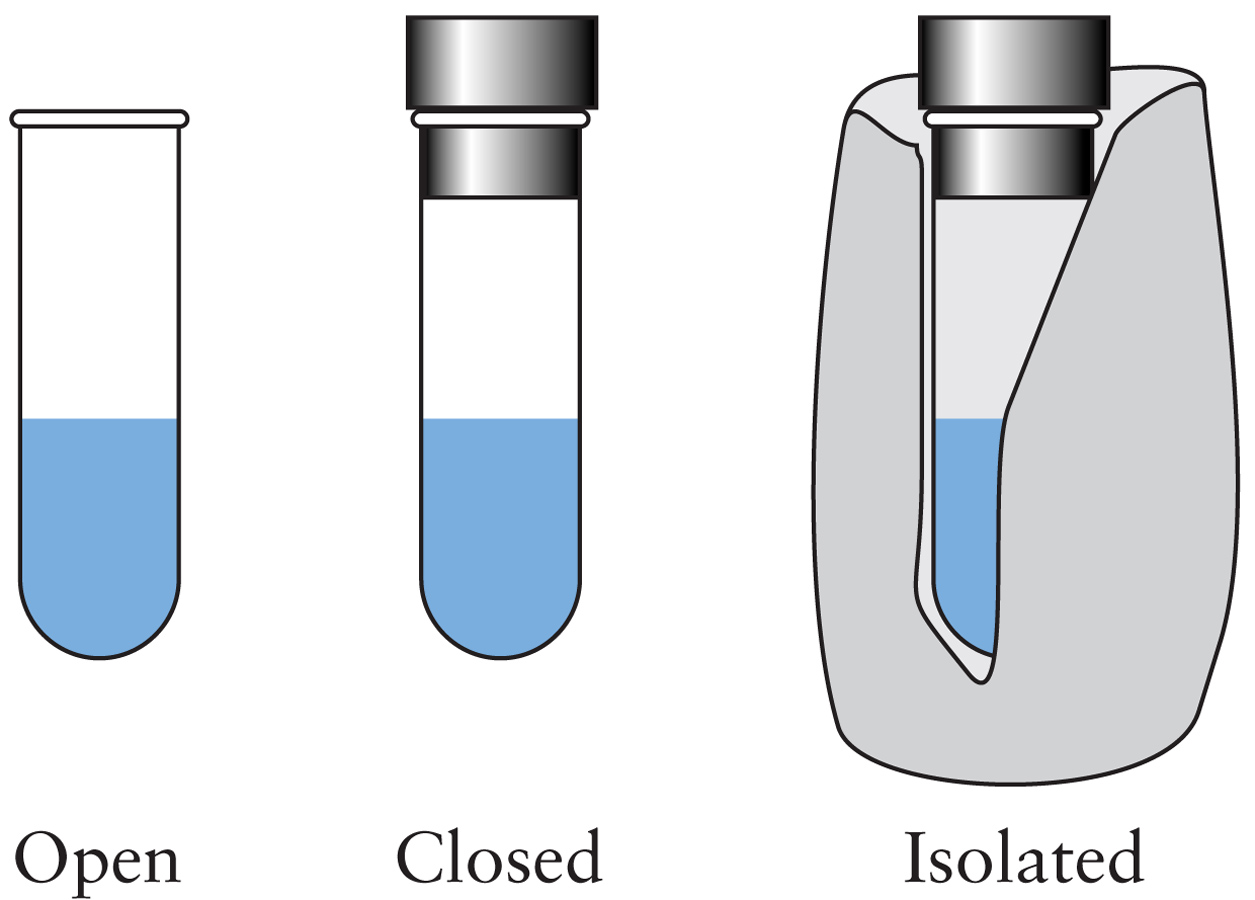
- For an isolated system: ΔUsystem = 0 - For a closed system: ΔUsystem = q + w - For a closed system at constant volume with only PV work: ΔUsystem = qv
|
|
|
|