Standard Formation Reaction
A chemical reaction in which one
mole of a substance
in its standard state
is made from it's elements
in their standard states.
Standard State
: Pure substance in most stable thermodynamic state at a pressure of 1 bar (~ 1atm) and a fixed temperature (often chosen to be 25oC).
Standard Enthalpy of Formation
: The enthalpy change for a standard formation reaction,
ΔHof
.
|
|
|
Using Standard Enthalpies of Formation:
|
|
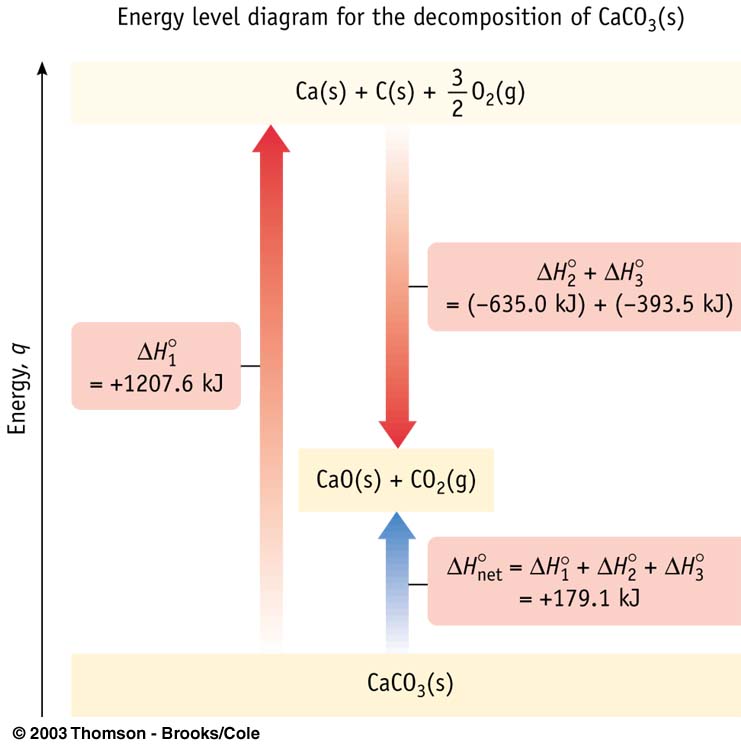
Equivalent to turning reactants into elements then forming products from these elements.
|
|
Example: CaCO3(s) + heat –––> CaO(s) + CO2(g)
|
 |
|
|
|
|