Reactions Summary
ΔG = ΔH – TΔS < 0 Spontaneous
Constant T and P
|
|
|||
|
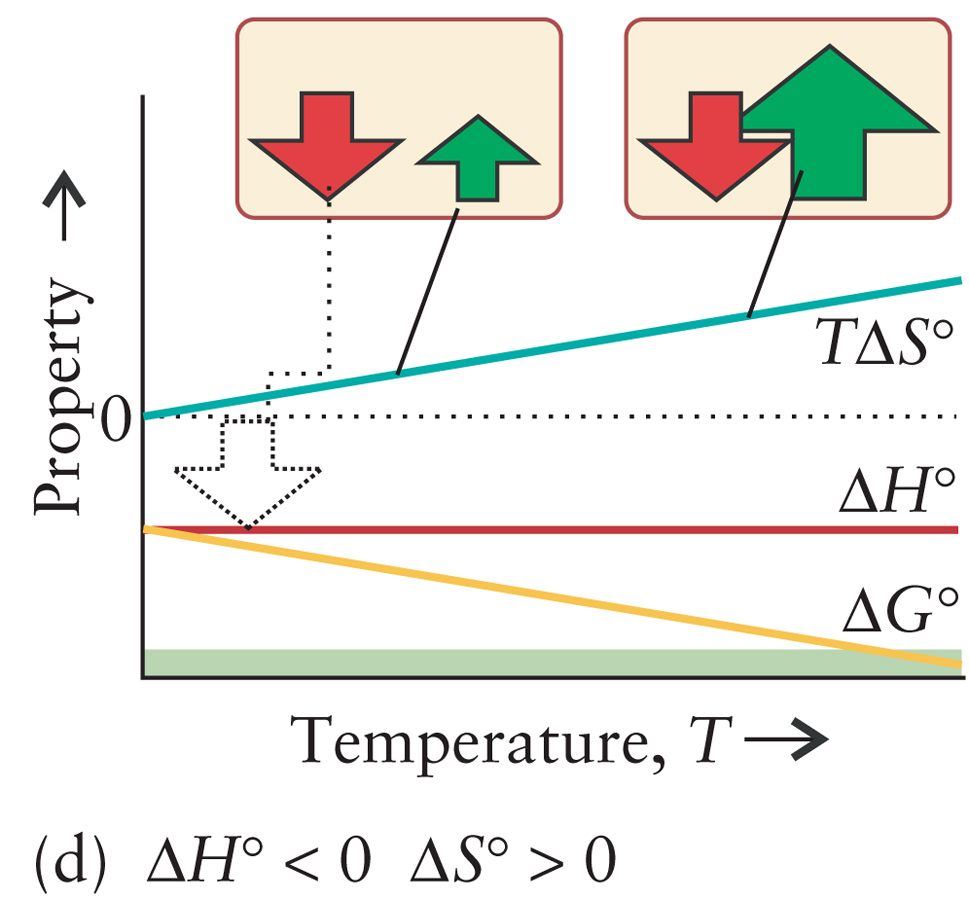
Burn Sugar
C6H12O6(s) + 6O2(g) ---> 6CO2(g) + 6H2O(l) |
ΔH < 0
ΔS > 0 ΔG < 0 All Temperatures
|
|
|
|
|
|||
|
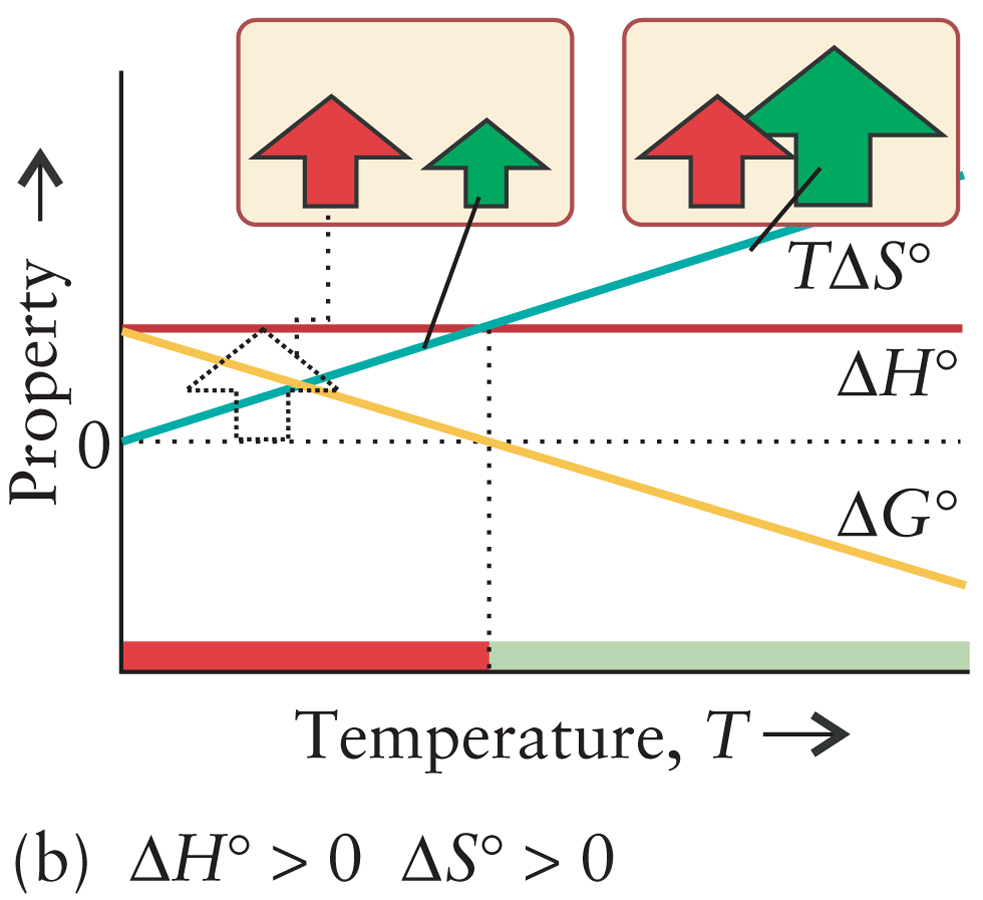
Freeze Liquid
|
ΔH < 0
ΔS < 0 ΔG < 0 Low Temperatures |
|
|
|
|
|||
|
Crystallize Supersaturated Solution |
ΔH < 0
|
||
|
|
|||
|
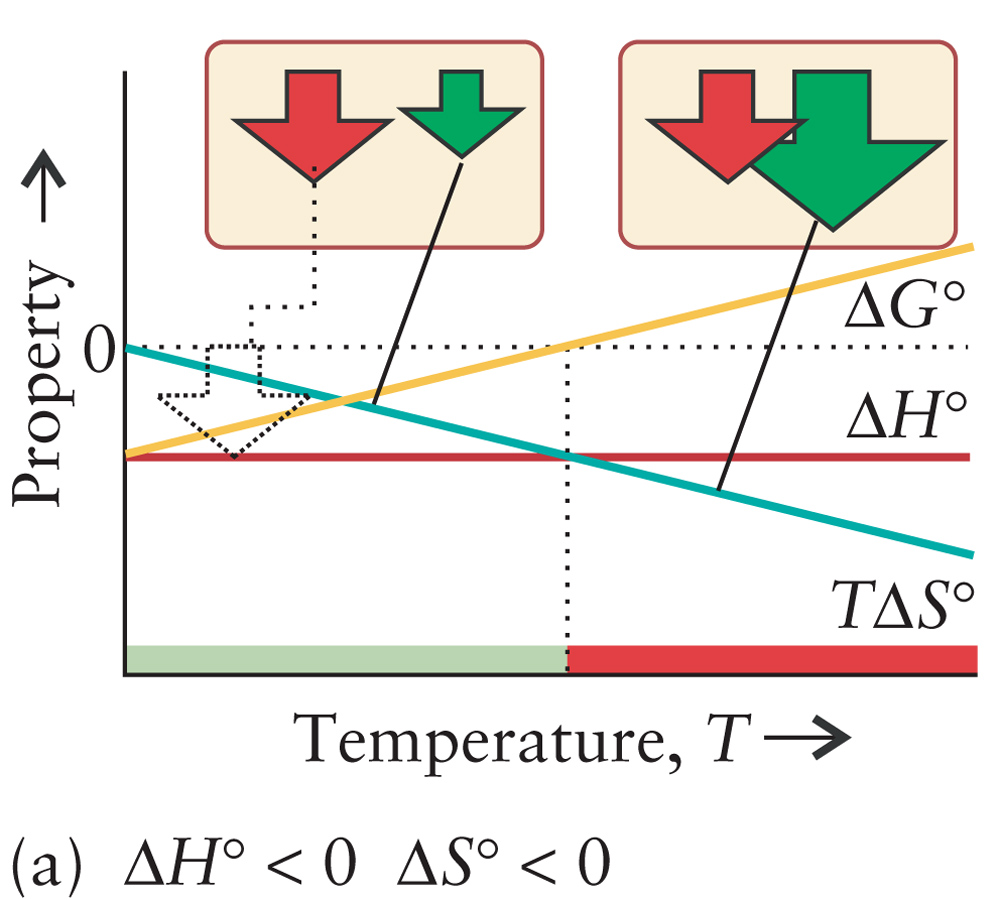
Slurry from two solids
Ba(OH)2(s) + NH4NO3(s) ---> H2O(l) + Ba2+(aq) + NO3–(aq) + NH3(aq) |
ΔH > 0
ΔS > 0 ΔG < 0 High Temperatures |
|
|
|
|
|||
|
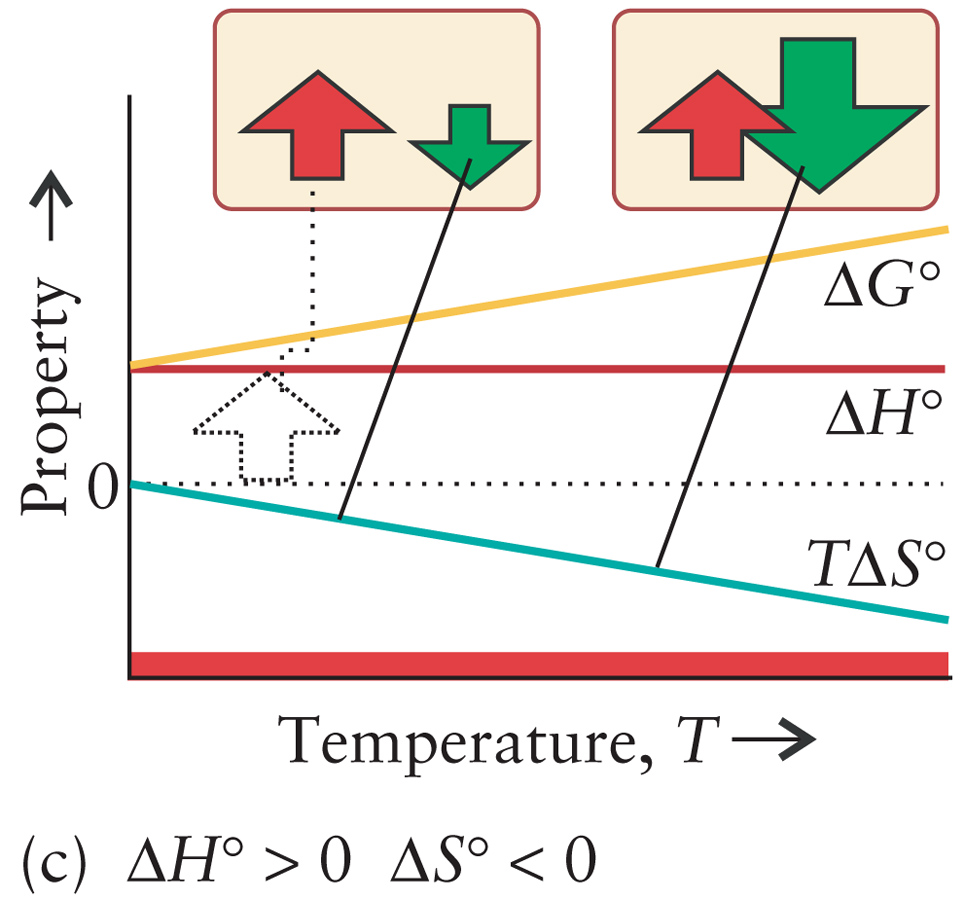
Un-Burn Sugar
(Photosynthesis)
6CO2(g) + 6H2O(l) ---> C6H12O6(s) + 6O2(g) |
ΔH > 0
ΔS < 0 ΔG < 0 No Temperature Must be driven by external influence. |
|
|
|
|
|||