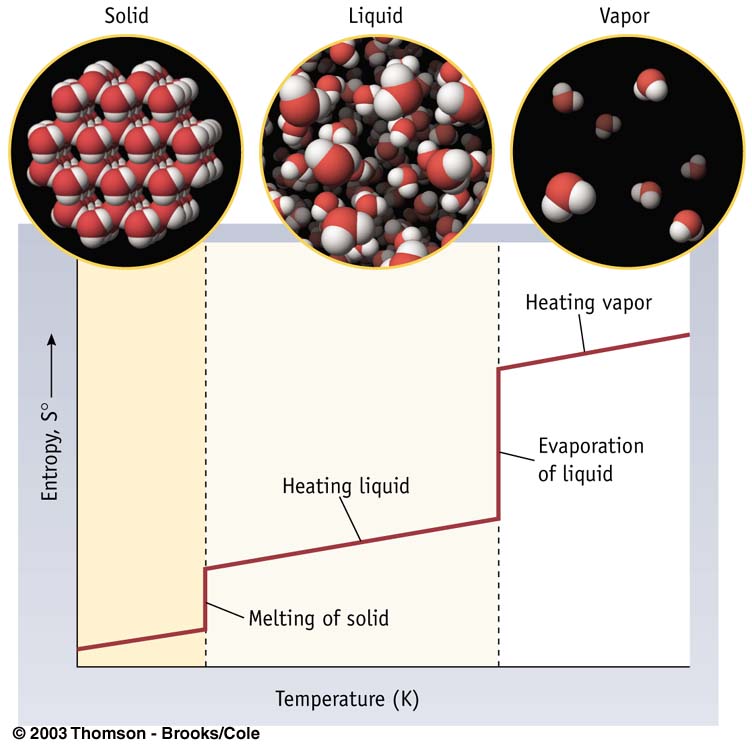
Entropy of Phase Change
Constant Temperature
| ΔSphase = |
q(reversible)
|
= | ΔH(phase) |
|
T
|
T
phase
|
Reversible Process if takes place at NBP, NMP or NSP.
|
|
||
 |
|
|
|
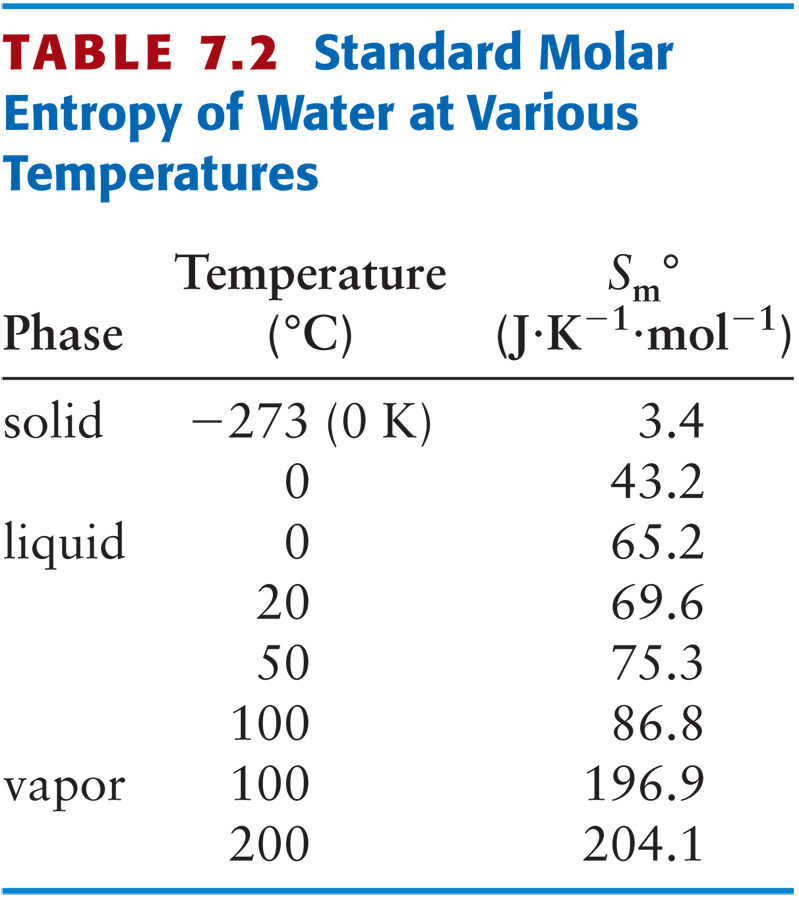
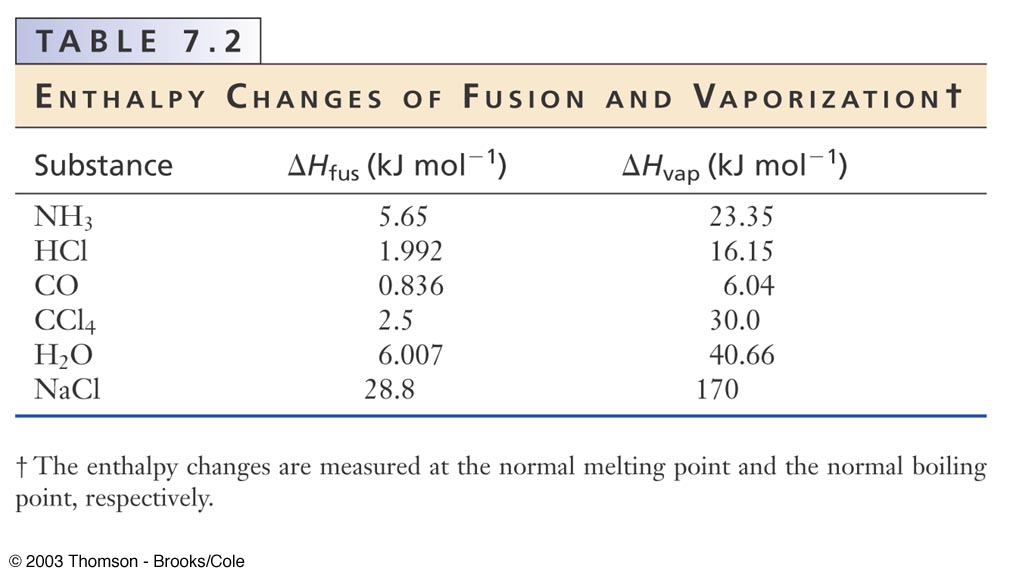
Calculate the entropy change of 18.02 g of water when it is condenses at its boiling point.
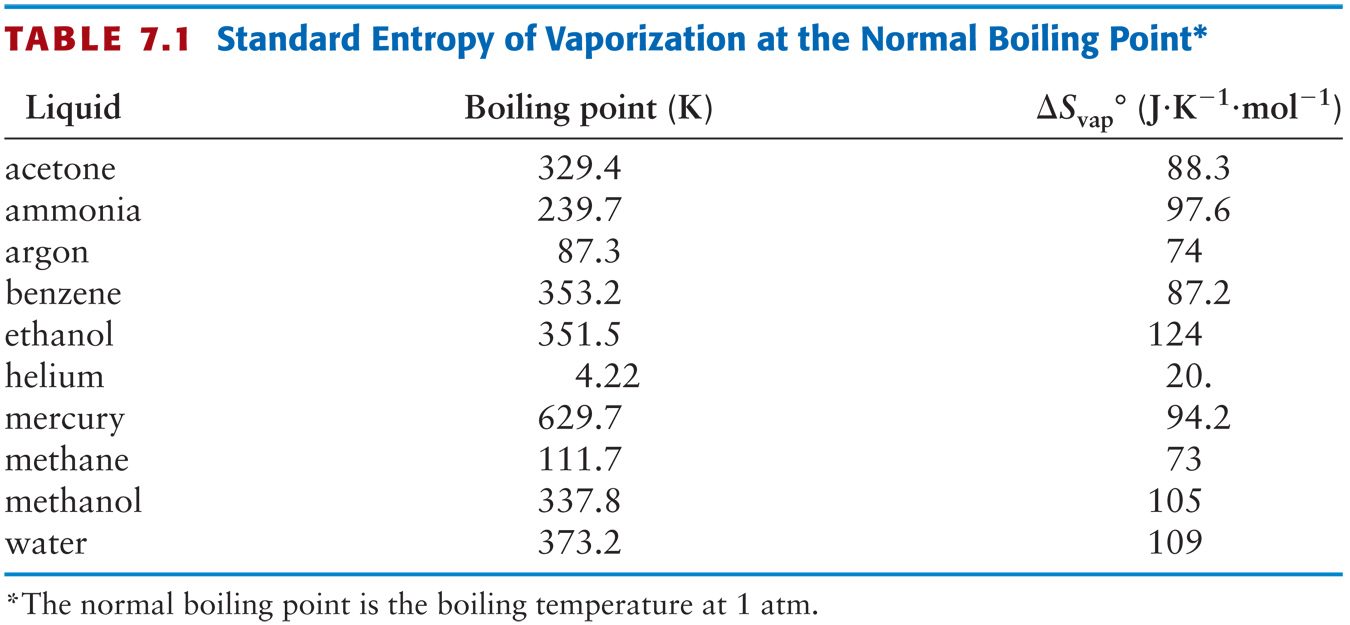
Calculate the entropy change of 1 mole of ammonia when it melts at one atmosphere, NBP = –330C. |
|
|
|
||
|
|
||