Chemical Equilibria
– Key Ideas
|
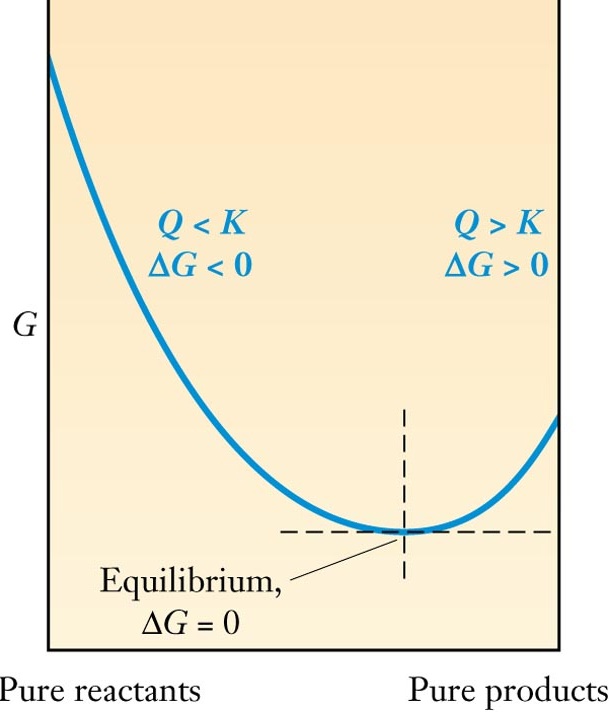
Reactions proceed until composition of rxn mixture
corresponds to minimum Gibbs Free Energy. |
|
|
Composition at minimum G is described by Equilibrium Constant, K. K - characteristic of reaction K - depends on Temperature Tells how much product can form. Change rxn conditions, can predict how system will reach equilibrium. |
|