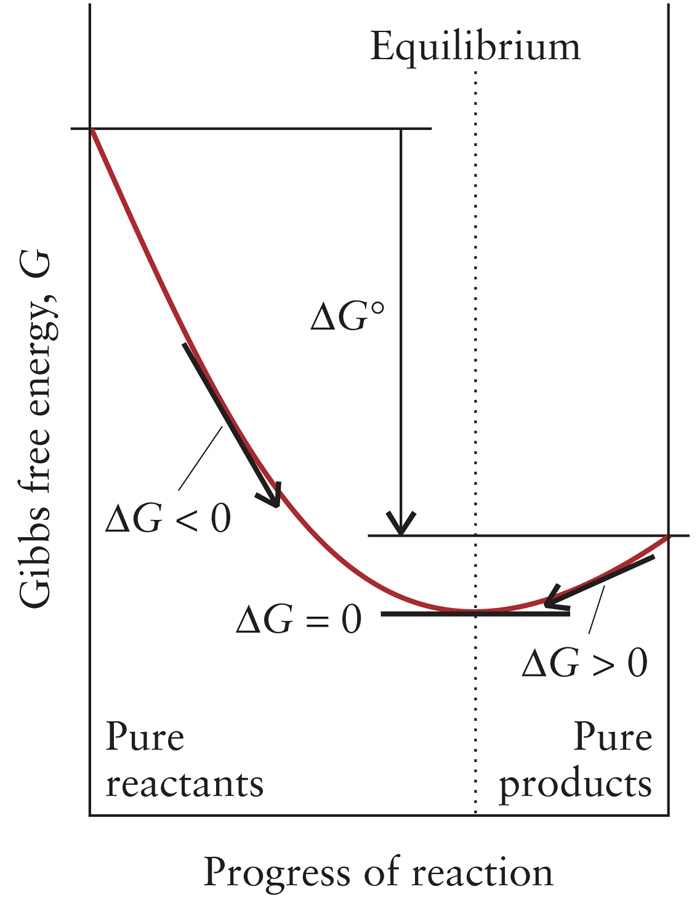
Reactions proceed until composition of rxn mixture (given by Q)
corresponds to minimum Gibbs Free Energy.
ΔG changes throughout reaction because Q changes.
ΔG = ΔG0 + RT ln Q
 |
|
|
ΔG0 < 0 K > 1 Product–favored at equilibrium |
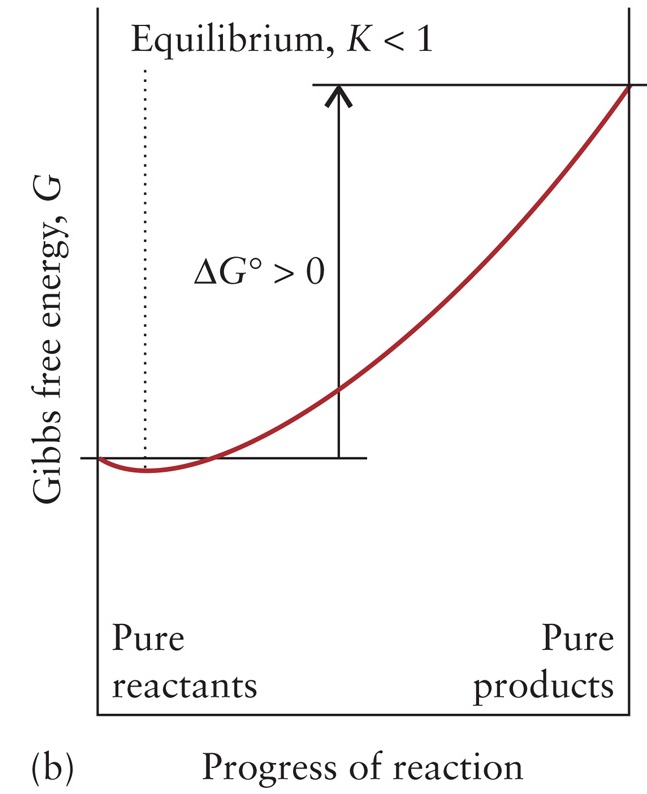
ΔG0 > 0
K < 1 Reactant–favored at equilibrium |