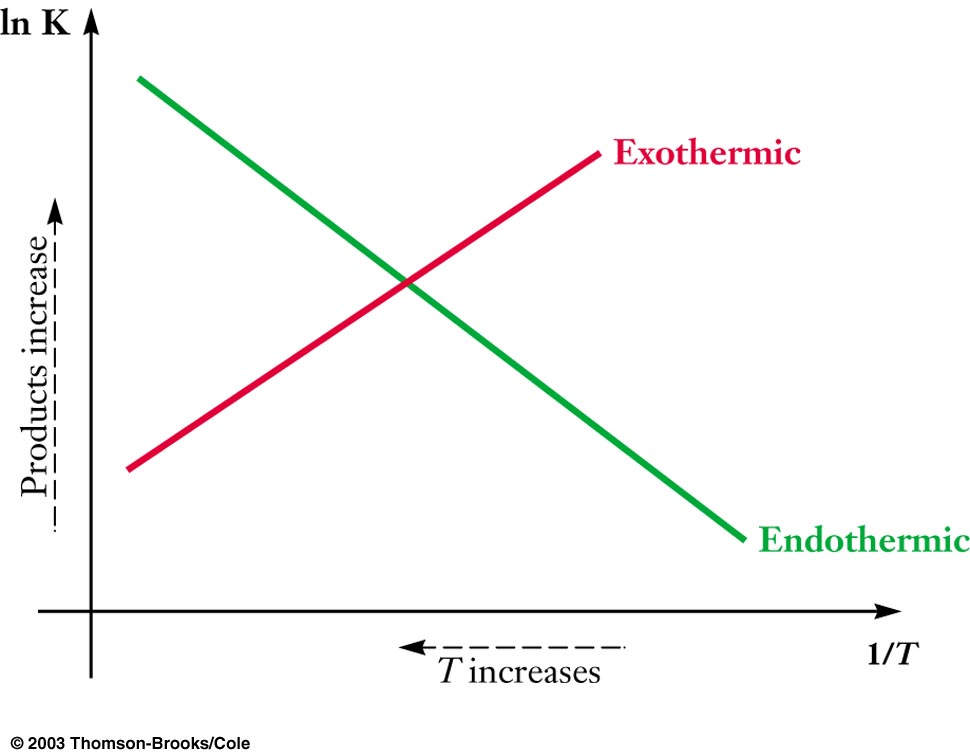
Equilibrium Constant and Temperature
|
|
|||
| Increase T |
ΔHrxn > 0
Endothermic |
|
K increases |
|
ΔHrxn < 0
Exothermic |
|
K decreases | |
|
|
|||
ln(K2/K1) = – (ΔHorxn/R) (1/T2 + 1/T1)
|
|
|
|
|