

| Hydrogen bonds are weaker than covalent bonds, but are nevertheless a stabilizing interaction. The simplest way to view a hydrogen bond is by thinking about simple electrostatics. The peptide bond is a good example of how resonance structures can create partial charges on different parts of a molecule. The electrostatic interaction of one positively charged center with one negatively charged center is favorable. |
| Note that alternate resonance forms can also describe the hydrogen bond! Here we see a form which places a formal bond between the original amide hydrogen (donor) and the carbonyl oxygen (acceptor). This is simply a different representation of the electronic distribution - the molecular structure remains unchanged (as it should for an alternate resonance form). | 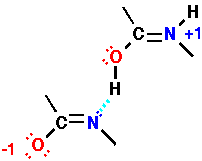
|
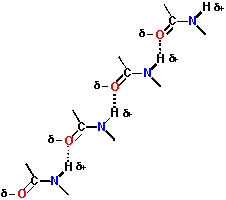
| The nature of the amide bond allows interconnected, repeating structures to be formed. This occurs in structures such as alpha-helices and beta-sheets. |