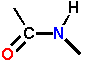
| is shown as
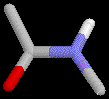
| 
 |
Amide bonds are resonance hybrids
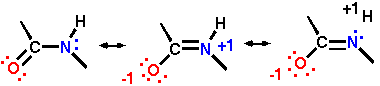
Note that the result is a partial double bond between C and N, and the placement of partial negative charge on oxygen and partial positive charge on nitrogen (and out onto the hydrogen as well, there is a third resonance structure to do this!).
Which is also explained by sp2 hybridization
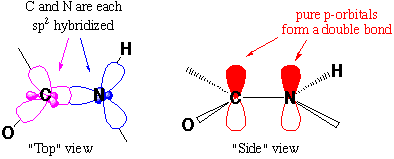
The geometry of amide bonds can also be explained by valence bond hybrid orbitals.







