|
|
| |
|
Principal Research Interests
|
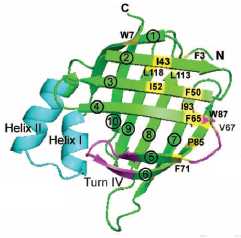
Protein Folding
|
|
|
The protein folding problem, namely how amino acid sequence determines the
three-dimensional structure of a protein, is not fully understood despite
many years of effort. We are addressing this problem in a variety of ways
in our laboratory: We study the conformational preferences of model peptides
in order to explore how local sequence guides folding. We are also carrying
out detailed studies of the in vitro folding of a predominantly β–sheet protein
with a very simple topology. Methods we use in all of our folding work include
circular dichroism, fluorescence,and nuclear
magnetic resonance.
|
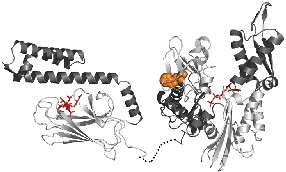
Chaperones
|
|
|
We are also interested in how a protein folds in vivo. In recent years,
a class of proteins called molecular chaperones has been found to facilitate
protein folding in vivo. We are addressing several questions concerning
chaperones: How do they recognize and bind incompletely folded polypeptides?
Do different classes of chaperones bind to their substrates in distinct
ways? How do chaperones interact with their co-chaperones? Is the mechanism
of chaperone-mediated folding different from that of the isolated protein?
|
|