 |
|||
 |
|||
Nickel Trafficking |
|||
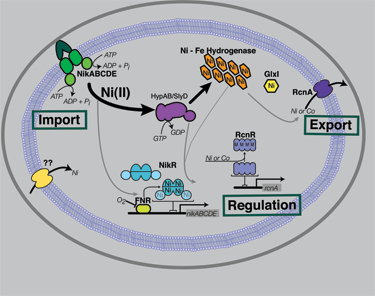 |
The Maroney Group is a interdisciplinary group that focuses on the structure and function of metalloenzymes. The main interest within the lab is metallobiochemistry. Understanding why nickel is used in redox active enzymes and how the cells is able to control the intracellular levels of nickel are studied extensively using both synthetic and biochemical approaches.
|
||
Nickel Superoxide Dismutase |
|||
Nickel Superoxide Dismutase (NiSOD) is an enzyme that catalyzes the dismutation
of superoxide to molecular oxygen and hydrogen peroxide. There are four known SOD's
that are characterized by their metal center; 1) Cu/Zn, 2) Fe, 3) Mn and 4) Ni.
We are interested in studying NiSOD to determine the mechanism by which NiSOD
operates as well as understand how nature tunes the redox potential of the Ni(II/III)
couple which lies above 2 V in aqueous solution. To gain insight the lab utilizes
site-directed mutagenesis and metal substitution to alter the properties of the enzyme.
These different samples are studied with a number of different techniques to determine
the metal coordination environment, the catalytic activity and reduction potential.
|
 |
||
[NiFe]-Hydrogenase |
|||
 |
Every year the reality of the diminishing supply of hydrocarbon resources in the world becomes more daunting. This coupled with the desire to eliminate the ill effects of burning hydrocarbon fuels has brought about interest in alternative energy resources. Since hydrogen is an idealistic clean energy fuel, its sustainable production is in demand worldwide. Hydrogenases (H2ases), are enzymes that catalyze the deceptively simple chemistry of combining two electrons with two protons to form H2.
The Maroney Group is interested in understanding the fundamental mechanics of these enzymes. By using time resolved infrared (TRIR) spectroscopy, one can characterizes the various catalytically relevant intermediates. These insights would allow chemists to intelligently design hybrid catalysts that can covert water into H2 or efficiently consume H2 and produce electricity as a byproduct, another facet of our research. Preliminary designs involve affixing H2ase onto both inorganic and organic semiconducting electrodes for H2 production and consumption, respectively. |
||
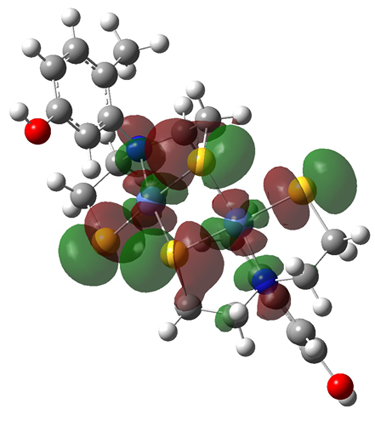
Synthetic Models |
|||
Our research involves using synthetic models to study the roles of tyrosines 9 and 62 in the dismutation of superoxide by nickel superoxide dismutase. Superoxide dismutases (SODs) carry out the dismutation of superoxide to molecular oxygen and hydrogen peroxide while alternating between the oxidized and reduced forms of the metal ion. NiSOD is the most recently discovered class of superoxide dismutases (SODs), and has no sequence homology with the other two classes of SODs (Cu/ZnSOD, Mn or FeSOD). The crystal structure of NiSOD shows that there are two tyrosine (Tyr 9 and 62) residues close to the metal center. The exact roles of these tyrosines in SOD catalysis are unknown, but they may play roles in redox/protection from radicals, regulating superoxide binding equilibria, proton donation, or stabilizing the enzyme structure by means of aromatic stacking. Synthetic models containing sulfur and nitrogen ligands coordinated to nickel as well as phenol moieties are being synthesized and will be studied to assess the roles of tyrosines in the NiSOD reaction. |
 |
||
