
Andrew K. Boal

1303 LGRT
boala@chem.umass.edu
(413) 545-5865
Education:
Ph.D. (Organic Chemistry) University of Massachusetts, Amherst; 1998 -
M.S. (Organic Chemistry, under David A. Shultz) North Carolina State University; 1996-1998
B.S. (Minor in English) North Carolina State University; 1992-1996
Research Interests:
Synthetic Organic Chemistry, Organic Materials Chemistry, Colloid/Surface/Interface Science, Molecular Devices, Supramolecular Chemistry, and Physical Biochemistry.
Current Research:
Molecular Recognition in Self-Assembled Monolayers on Gold Nanoparticles
The main research I am involved in centers around the preparation and investigation of molecular recognition element functionalized self-assembled monolayers on gold nanoparticles. Like gold surfaces, colloidal gold- gold particles whose size is in the nanometer regime- allows the surface formation of alkane thiol self-assembled monolayers. Unlike gold surfaces, these systems can be treated as large organic molecules: they are amenable to characterization techniques like NMR that cannot be done on surfaces and thus facilitate investigation of molecular recognition phenomena. Additionally, nanoparticles present many unique opportunities for supramolecular chemists unavailable in small molecule systems, as well as provide the opportunity do develop novel materials.
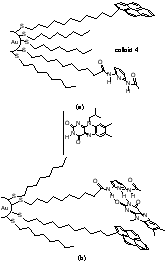
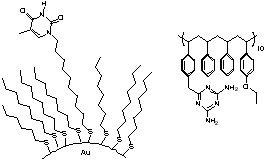
There are many questions presented in the exploration of this new form of host-guest interactions, including the availability of a recognition element functionalized surface for guest occupancy (e.g. will the element bury itself in the monolayer, will there be too much crowding to allow for guest occupancy, etc.). Our first system involved the preparation of a colloid functionalized with diaminopyridines, and we found that flavin interacted with it in much the same way that it does with a diaminopyridine that is free in solution.
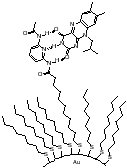
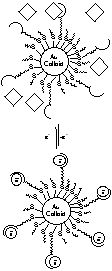
Figures showing the binding of flavin to a diaminopyridine bound to colloidal gold and the enhancement of recognition upon reduction of flavin.
We then began the exploration of the unique opportunities afforded to supramolecular chemistry in this novel system. An enthalpically driven process was employed to self-optimize a multivalent flavin binding site on a nanoparticle surface. In this experiment, flavin was first bound to the colloid by hydrogen bonding. Recognition was then enhanced as pyrene elements, which can p-stack with the flavin, moved around on the colloid surface to create the multivalent binding site.
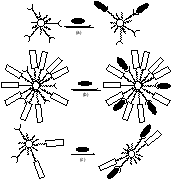
Figures showing binding to a monovalent and multivalent binding site, as well as self-optimization of a multivalent binding site.
Presently, this research is expanding down several paths. We are exploring the possibility of using these multivalent colloidal surfaces to bind different guests via different binding pathways. There are also questions involved in the strength of aromatic-aromatic interactions as a function of tether length; the enhancement in recognition by the addition of stacking elements was not as significant as was observed in our previous small molecule hosts. Incorporation of hydrogen-bonding moieties in the monolayer chain, such as amides, would have a major effect on monolayer stability and may allow for the formation of unsymmetericly functionalized surfaces both of nanoparticles and flat surfaces. There are also many extensions of binding site self-optimization, with possibilities ranging from the fabrication of binding sites from basic components which could recognize a variety of guests to constructing systems able to template their surfaces for the specific recognition of biomolecules. Also currently under investigation is the incorporation of flavin elements in the SAMs in attempts to develop novel biological model systems.
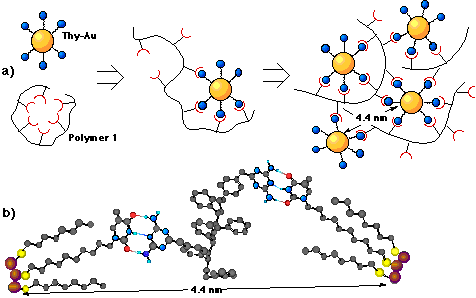
Self-Assembly of Nanoparticle Arrays via. Hydrogen Bonding
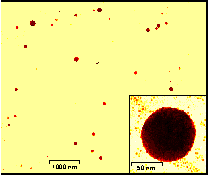
One of the main goals of this project is to develop molecular recognition based schemes by which nanoparticles arrays can be prepared. Our first result in this area involved the interaction of thyamine functionalized colloidal gold with a diaminotriazine functionalized polymer. Reaction of these two components in dichloromethane resulted in the formation of a dark brown precipitate. This solid was investigated by X-Ray scattering, in which we found that the average particle-particle separation was on the order of 4.7 nm. After modeling possible colloid-polymer interactions, we devised a scheme in which particles were glued together by a single polymer strand. We also found that this solid could be dissolved in polar solvents such as THF and MeOH, and on investigation of these solutions with TEM, we found that the solid was consisted of large spherical particles of 97±17 nm diameter, comprised of 3000 &endash; 7000 individual components. This represents a discovery of a novel nanoparticle array fabrication technique in that it creates highly ordered assemblies. We are currently investigating the properties of these assemblies; factors controlling array formation such as polymer structure, colloid structure, temperature, and solvent effects; the fabrication of arrays based on semiconducting, luminescent, and magnetic components; and the solution phase stability of these arrays.
Publications :
"A 'Building Block' Approach to Mixed-Colloid Ensembles through Electrostatic Self-Organization" Galow, T. H.; Boal, A. K.; Rotello, V. M. Adv. Mater. 1999, submitted
"Fabrication and Self-optimization of Multivalent Receptors on Nanoparticle Scaffolds" Boal, A. K.; Rotello, V. M. J. Am. Chem. Soc. 1999, in press
"Self-Assembly of Nanoparticles into Giant Spherical Arrays." Boal, A. K.; Ilhan, F.; Thurn-Albrecht, T.; Russell, T. P.; Rotello, V. M. Nature, 1999, submitted
"Divergent Monolayer Synthesis Using Acid Fluoride Functionalized SAMs." Niemz, A.; Jeoung, E.; Boal, A. K.; Deans, R.; Rotello, V. M. Langmuir, 1999, in press.
"Redox Modulated Molecular Recognition of Flavin by Gold Nanoparticle Bound Diaminopyridine" Boal, A. K. and Rotello, V. M. J. Am. Chem. Soc. 1999, 121, 4914.
"Structure-Property Relationships in Trimethylenemethane-Type Biradicals. 2. Synthesis and EPR Spectral Characterization of Dinitroxide Biradicals." Shultz, D. A.; Boal, A. K.; Lee, H.; Farmer, G. T. J. Org. Chem. 1999, 64, 4386.
"Preparation and Characterization of a Bis-Semiquinone - a Bidentate Dianion Biradical," Shultz, D.A.; Boal, A.K.; Driscoll, D.J.; Kitchin,J.R.; Tew, G.N. J. Org. Chem., 1995, 60, 3578-3579.
"Galvinitroxide - a New Triplet Biradical," Shultz, D.A.; Boal, A.K. Mol. Cryst. Liq. Cryst. 1995, 272, 75-79.
"Preparation of Paramagnetic Ligands for Coordination-Complexes and Networks With Interesting Magnetic Properties," Shultz, D.A.;Boal, A.K.; Driscoll, D.J.; Farmer, G.T.; Hollomon, M.G.; Kitchin, J.R.; Miller, D.B.; Tew, G.N. Mol. Cryst. Liq. Cryst. 1997, 305, 303-310.
"The Biradical, Bis-(3,5-di-tert-butyl-4-phenoxyl)methyleneadamantane, Exhibits Matrix-Dependent EPR Spectra Suggesting Rotamer Bistability with Differential Exchange Coupling," Shultz, D.A.; Boal, A.K.; Farmer, G.T. J. Am. Chem. Soc. 1997, 119, 3846-3847.
"The Effect of Aliphatic Amine Bases on the Aggregation of Alkali Metal Salts of 3,5-Di-tert-butylsemiquinone (3,5-DBSQ)," Shultz, D.A.; Boal, A.K.; Campbell, N.P. Inorg. Chem. 1998, 37, 1540-1543.
"Synthesis of Bis(semiquinone)s and Their Electrochemical and Electron Paramagnetic Resonance Spectral Characterization," Shultz, D.A.; Boal, A.K.; Farmer, G.T. J. Org. Chem. 1998, 63, 9462-9469.
Return to group members page
Return to group homepage