
Trent H. Galow

1309LGRT
galow@chem.umass.edu
(413) 545-4865
Education:
Ph.D. University of Massachusetts, MA; 1996-
1st Class Hons. B.Sc., University of Huddersfield, UK, 1996
Berk Pharmaceuticals, Eastbourne, UK, 1994
Honor and Awards:
Marriott Prize, Royal Society of Chemistry, 1995
W.E. Scott Award, University of Huddersfield, 1996
Graduate of the Royal Society of Chemistry
Research Interests:
Organic synthesis, molecular recognition, silicate and polymeric materials.
Research:
One area of my research focuses on novel silicate-based systems. Silicate-based materials such as sol-gels and silica colloids are of great interest in industry and academia as a result of their incredible versatility and function. These systems can be prepared in a straightforward manner from the hydrolysis of the commercially-available ormosils (with either pre- or post-functionalization), providing novel materials with interesting physicochemical properties. We demonstrated, for example, how a sol-gel could be used to model aromatic-aromatic interactions found within flavoenzyme binding sites. By incorporating flavin mononucleotide (FMN) into a sol-gel, simultaneously with increasing equivalents of an anthracene derivative, we obtained efficient recognition within the silicate matrix (Figure 1). Furthermore, this recognition process was enhanced relative to that observed in solution, resulting from the silicate matrix preorganizing the binding site.
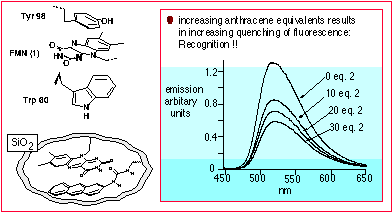
Figure 1: Top left picture depicts the aromatic interactions found within a flavoenzyme. Tyr and Trp are tyrosine and trytophan residues, respectively. Bottom left illustration shows the system we engineered to model this effect. The graph shows the result of increasing anthracene equivalents: a decrease in fluorescence intensity of FMN is observed.
A sol-gel project that I am currently researching is the development of an environmentally benign catalytic system. FMN (vitamin B2) is responsible for a wide variety of biocatalytic oxidations, such as amines to imines, sulfides to disulfides, dehydrogenation of lipid esters, D-amino acids and NAD(P)H. We are trying to utilize flavin's catalytic prowess to create a totally green catalytic system based on the concept shown in Figure 2.
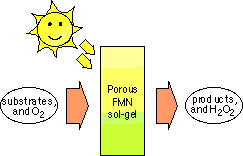
Figure 2: Schematic showing the basic concept behind the catalytic system. The process is driven by both sunlight and oxygen.
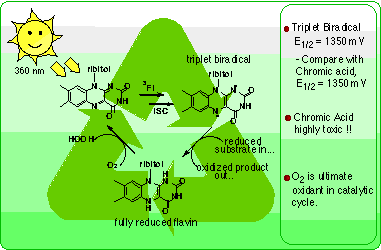
At 360 nm (sunlight!), flavin undergoes excitation and, via intersystem crossing, forms a triplet state biradical (Figure 3). This flavin biradical has a redox potential of 1297 mV, almost as high as chromic acid (1350 mV), and therefore becomes a powerful oxidizing agent. After oxidizing a substrate, the reduced flavin can be reoxidized by molecular oxygen, completing the catalytic cycle. This provides a totally green system driven by sunlight and oxygen. In solution, FMN excitation leads to decomposition. This can be circumvented by incorporation of FMN into a sol-gel providing isolation.
Publications:
"Communication of Electronic Information over Extended Distances. An Experimental and Density Functional Investigation" R. Deans, A. Cuello, T. Galow, M. Ober, V. Rotello, Submitted to J. Chem. Soc.,Perkins Treans. 2., 1999
"A 'Building Block' Approach to Mixed-Colloid Ensembles through Electrostatic Self-Organization" T. Galow, A. Boal, V. Rotello, Advanced Materials, in press
"Recognition and Encapsulation of an Electroactive Guest within a Dynamically Folded Polymer", T. Galow, F. Ilhan, G. Cooke, V. Rotello, J. Am. Chem. Soc., in press
"Flavins as Modular and Amphiphilic Probes of Silica Microenvironments" M. Greaves, R. Deans, T. Galow, V. Rotello, J. Chem. Soc., Chem. Comm., 1999, 785-786.
"Fluorocarbonylferrocene. A Versatile Intermediate for Ferrocene Esters and Amides", T. Galow, J. Rodrigo, K. Cleary, G. Cooke, V. Rotello, J. Org. Chem., 1999; 64(10); 3745-3746
"Model Systems for Flavoenzyme Activity. Aromatic Stacking in Silicate Matrices" M. Greaves, T. Galow, V. Rotello, J. Chem. Soc., Chem. Comm., 1999, 169-170.
Return to group members page
Return to group homepage